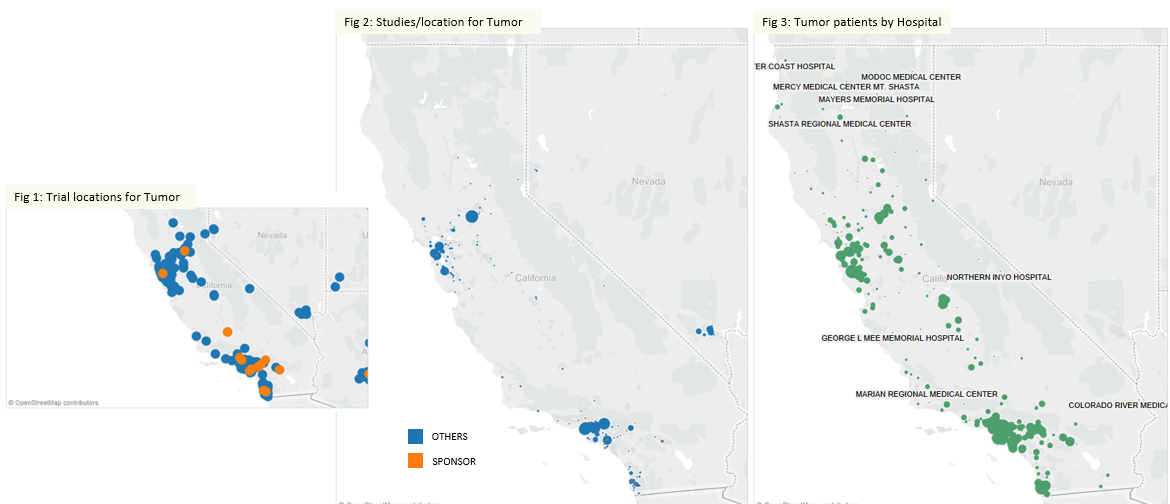
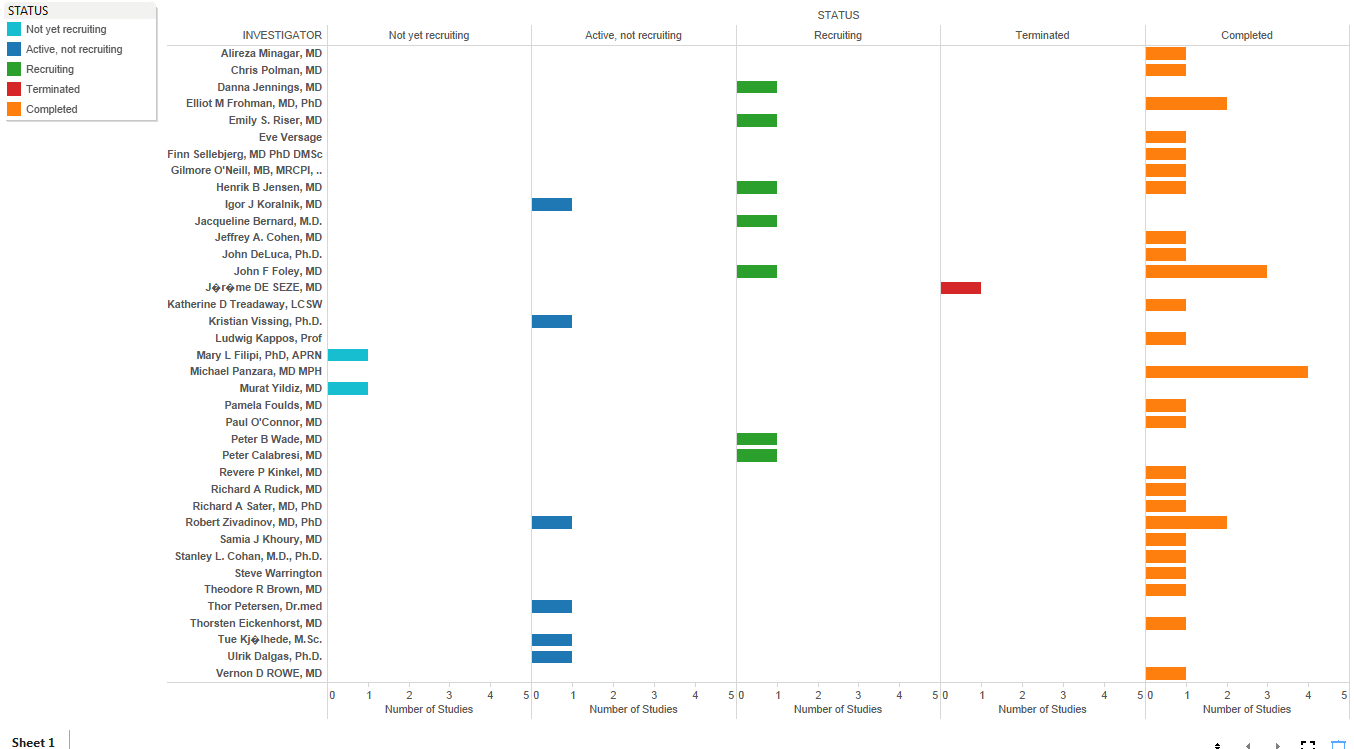
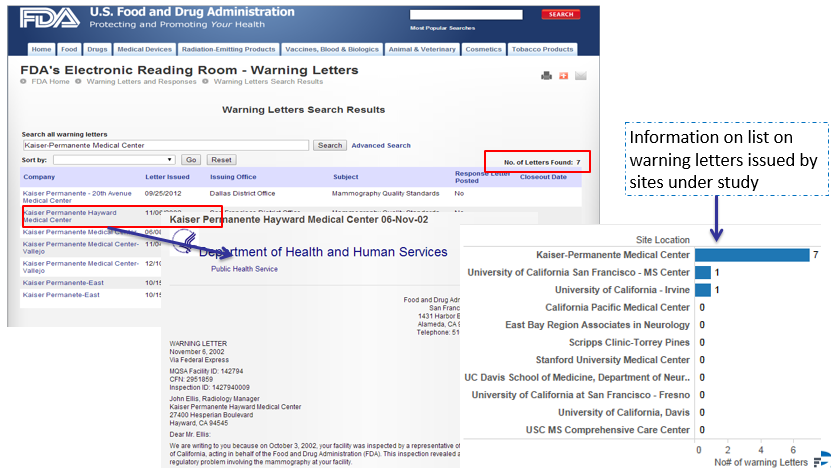
Sandeep Kishore, HCL Technologies, explores digitalisation and the role that a Chief Digital Officer can play in leading digitalisation projects.
Everywhere you look businesses are being reinvented and rejuvenated, utilising new technologies and tools in an effort to tap into the digital advantage. This process of digitalisation is as unstoppable as it is irreversible; businesses are investing heavily to stay afloat in the new digital age. As such, digitalisation has moved way beyond high-concept discussions at the board level, to the creation of detailed roadmaps and the implementation of new ways of working and service delivery. This change is bringing with it the ability for businesses to create unique customer-facing experiences, whilst driving greater back-office efficiencies.
Spending on digitalisation over the remainder of the decade is forecast to be enormous; contributing to over 80% of incremental IT expenditure. Indeed, IDC estimates that spending on cloud, social, mobile and analytics technology will reach around $940 by 2017. The compound annual growth rate is set to be three times greater in comparison with spend on traditional IT over the same period. This level of investment indicates that digital technologies will clearly have a major direct impact on businesses in the coming years.
Playing with the new kids on the block
Those that are most well suited to thrive in the digital era are perhaps unsurprisingly those businesses that were born digital and have none of the legacy infrastructure of more well-established companies. The savviest enterprises are looking to tap into this expertise, by working with native digital companies to better deliver unified, reliable and seamless customer experiences across a multi-platform environment. Furthermore, this approach can help to ensure better integration with suppliers and partners, such as payment providers; which delivers further operational efficiencies.
For example, in the media and entertainment industry, Netflix has emerged as an alternative to regular satellite and cable television services. For a fraction of the cost of the traditional alternatives, subscribers are able to watch any number of shows, at any time, on any screen. The success of this model speaks for itself; Netflix currently has 44 million subscribers globally. Low pricing is of course a key factor in this success, but just as importantly, Netflix is using analytics to deliver customised recommendations to each subscriber, suggesting additional content they may enjoy based on their viewing history. All this is made possible by cloud computing; which enables Netflix to deliver a previously impossible to achieve standard of service at such a low price without jeopardising its profits.
Enter the Chief Digital Officer
Of course, it is no mean feat for a traditional enterprise to achieve full digitalisation. There must be a significant commitment from within every level of the business to ensure that all teams are working towards a shared vision and goals. This means that the CEO and board-level executives must have direct visibility over the process to ensure the necessary levels of support and investment are made available.
In order to facilitate this process more effectively, many organisations have created the new role of Chief Digital Officer (CDO) to oversee digitalisation. The CDO is responsible for developing a clear roadmap for the enterprise in its transition to the digital era; clarifying the role of every business unit and securing the buy-in from each individual.
As you would expect, IT has a significant role to play on the road to digitalisation for any organisation. The IT team will be responsible for modernising applications and integrating all data across the enterprise. They must also enable access to the corporate network and resources through mobile devices, build cloud platforms and enhance the security architecture to enable all of this without introducing new vulnerabilities. It is also critical to ensure that users receive a consistent experience across all enterprise services; so digital architects and product strategists too have a significant role to play.
Of course, digitalisation is not a silver bullet to solve all of an enterprise’s problems. There is also no simple, one-size-fits-all approach to rolling out a digital strategy. Those beginning the journey to digitalisation must consider the individual circumstances of their business and identify the desired impacts and how they can best be achieved. This is a strategic decision and will need the involvement of the highest echelons of the company. It is also important to consider which functions can deliver the greatest value in the short-term, who will take the lead and whether it will be necessary to hire new talent to better oversee digital best practices.
It must also be decided whether to start by digitalising customer-facing services to improve their experience, or back-office systems to create greater efficiencies. Perhaps most importantly, businesses must consider how the culture of the organisation will need to adapt in order to deliver the best impact from its digital revolution.
Digitalisation is a continuous journey and will be a long-term commitment for those embarking upon it. However, it has huge potential to deliver significant business benefits and create strong competitive advantages in today’s challenging environment. Digitalisation is therefore not so much a choice as an imperative. Ultimately, it is a simple evolutionary principle; businesses will either adapt or die as the digital age continues to surge forwards.
By Sandeep Kishore, Corporate Vice President for Life Sciences, Healthcare, and Public Services, HCL Technologies